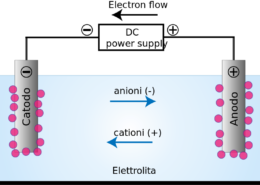
Je! Seli za Electrolytic Zinaweza Kugawanya Voltage?
Seli za electrolytic zinaweza kugawanya voltage, lakini hazina ufanisi kama seli za photovoltaic katika kufanya hivyo. Hii ni kwa sababu seli za elektroliti hutumia elektrodi ya chuma kuunda mkondo wa umeme ilhali seli za photovoltaic hutumia semiconductor kuunda mkondo wa umeme.. Electrode ya chuma katika seli ya electrolytic inaweza kupunguza kasi ya mchakato wa kuunda umeme, ambapo semiconductor katika seli ya photovoltaic inaweza kuharakisha mchakato.
Kitaalamu inawezekana kwa seli za Electrolytic kugawanya voltage, lakini hii haifanyiki kwa vitendo. Sababu kuu ya hii ni kwamba seli zingekuwa zisizo thabiti na hazingeweza kufanya kazi iliyokusudiwa. Zaidi ya hayo, si salama kuwa na tofauti kubwa za voltage kati ya seli – hii inaweza kusababisha ajali hatari.


Acha jibu
Lazima Ingia au kujiandikisha kuongeza jibu jipya.