How do you seperate alcohol from water
Water and alcohols have similar properties because water molecules contain hydroxyl groups that can form hydrogen bonds with other water molecules and with alcohol molecules, and likewise alcohol molecules can form hydrogen bonds with other alcohol molecules as well as with water.
Seperation of alcohol from water
To separate a mixture of alcohol (ethanol) and water, you can use a process known as fractional distillation. This technique relies on the fact that the compounds in the mixture have different boiling points. Since ethanol boils at a lower temperature (78.5 degrees Celsius, or 173.3 degrees Fahrenheit) than water, the alcohol vaporizes while most of the water remains a liquid. A good distillation column will produce a mixture of 95 percent alcohol and 5 percent water. This ratio represents the most pure form of ethanol possible with distillation and is widely accepted as an industry standard.
- Pour the ethanol/water mixture into the round-bottom flask.
- Assemble the fractional distillation apparatus by attaching the fractioning column to the round-bottom flask. Attach the condenser to the fractioning column and place the distillate-capturing flask under it to capture the distillate.
- Place the Bunsen burner below the round-bottom flask and heat the mixture to above the boiling point of ethanol (about 80 degrees C).
- Maintain the mixture at a constant temperature until the boiling has ceased. At this point, you have completed distillation.
What is Fractional Distillation
Fractional distillation is the process of separating a substance into its parts (or fractions), taking advantage of different vapor pressure properties of those substances. Fractional distillation is often used as a synonym with “distillation” because distillation always takes advantage of a difference in boiling points of component substances for separation.
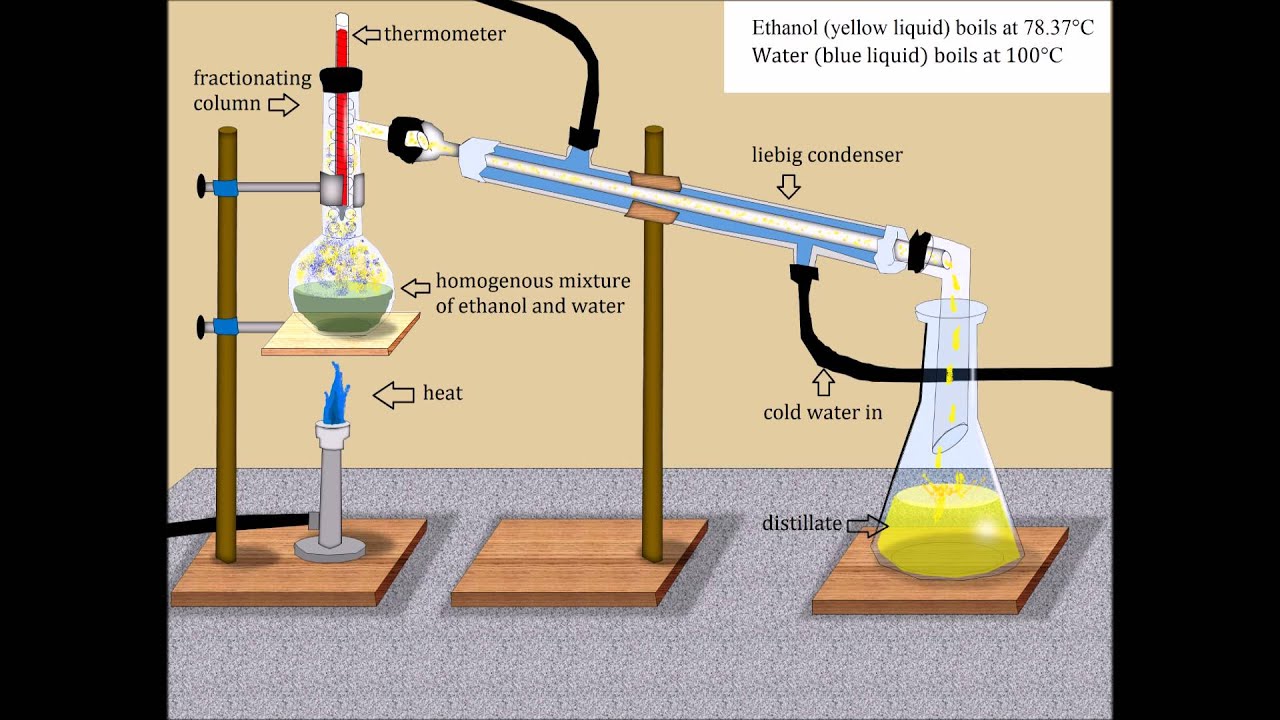
fractional distillation of alcohol and water
The basic steps to distillation are:
- Add heat to a liquid mixture with two or more main substances; for example, a water and ethanol mixture.
- As the liquid heats, components with the lower boiling points will begin to vaporize and rise through the column. In the water/ethanol example, ethanol will boil off first (BP 78° C, compared to water (BP 100° C). However, the vapor rising will still contain some molecules of the other substances. Vapor gets purer the higher it rises in the column, as heavier molecules “fall off” and turn back to liquid.
- As vapor rises in the distillation column, heavier molecules will condense back into liquid and “rain” back down. At any given point in a fractionating column, vapor will be rising, liquid will be falling, and molecules will be mixing. Columns naturally have certain “stages”; a stage is an area in the column with a similar amount of molecules of each type of substance (i.e. a general certain percentage of water and ethanol). Columns are designed to be tall enough to achieve a certain percentage separation, by finding the minimum number of required stages.
- Vapor reaching the top of the column (distillate) is collected into an industrial condenser (a big chiller), which cools the vapor back into a liquid, and piped to tank or storage.
- Substances remaining in the column continue the process of distillation, until the desired purity is reached. Some columns are a continuous process (most common), where new base solution is added continually. Others are batch systems, where the base is removed when a desired separation is achieved. In many systems, solution is recirculated several times to make sure substances are properly separated.
What is described above is the basic process of distillation. A distillation column is sometimes referred to as a fractional distillation column in industry. Columns that only separate two substances can be called fractionating columns. A fractional distillation system usually achieves several different products at multiple points within the column. As substances rise, mixtures can be pulled at various stages, and condensed.
Credit:
https://www.epicmodularprocess.com/blog/fractional-distillation


Leave an answer
You must login or register to add a new answer.