Difference between hydrogen bond and metallic bond
What is Hydrogen Bond?
Hydrogen bond is a form of intermolecular force that occurs when hydrogen bonds with highly electronegative elements such as nitrogen, oxygen or fluorine. In such a molecule, the negatively charged atom has a partially negative charge, and hydrogen has a partially positive charge. The relatively charged parts of the molecules strongly attract each other, like the poles of the magnets.
What is Metallic Bond?
Metallic bond occurs between atoms of a metal. The outermost electrons of the metal atoms become dislodged or “delocalized.” At this point the delocalized electrons do not belong to any particular atom but are shared as a communal “electron pool.” The positively charge nuclei of the atoms are all attracted to these electrons, which holds a piece of metal together.
Differences between Metallic and Hydrogen Bond
Metallic Bond
Metals are characterised by bright, lustre, high electrical and thermal conductivity, malleability, ductility and high tensile strength. A metallic crystal consists of very large number of atoms arranged in a regular pattern. Different model have been proposed to explain the nature of metallic bonding two most important modules are as follows:
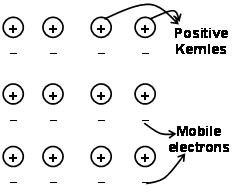
The forces that hold the atoms together in a metal as a result of the attraction between positive ions and surrounding freely mobile electrons are known as metallic bonds.
Through the electron sea predated quantum mechanics it still satisfactorily explains certain properties of the metals. The electrical and thermal conductivity of metals for example, can be explained by the presence of mobile electrons in metals. On applying an electron field, these mobile electrons conduct electricity throughout the metals from one end to other. Similarly, if one part of metal is heated, the mobile electrons in the part of the metals acquire a large amount of kinetic energy. Being free and mobile, these electrons move rapidly throughout the metal and conduct heat to the other part of the metal.
Conditions for Metallic Bond
Metal bond can be described as sharing free electrons between positively charged metal Ionic lattices. The structure of the metal bond is very different from the structure of the covalent bond and the ionic bond. In the metal bond, the valence electrons delocalize the S and p orbitals from the interacting metal atoms. That is, they do not revolve around their respective metal atoms, but around the positively charged nuclei of the interacting metal ions to form the”sea” of electrons. Then the electrons move freely in the space between the nuclei of the atom.
Keys are usually formed because individual atoms are unstable and bond formation creates a more stable structure.
All atoms have valence electrons: the number of groups of the periodic table tells you how many valence electrons have a specific element or metal (this differs from the d-block element, ie the transition metal).
Types of Metallic Bond
Metals are more liquid but not really bound in crystalline way. Ie. Some floating electrons around the closely associated atoms.Three states of matter exist.
Crystal- regular ordered array of atoms/molecules- required for structure determination through X-ray chrystalography
Liquid-i.e. Moving entities in close proximity ( I may be wrong but the way it was explained to me is that “solid” is not a state of matter. Metals are closer to liguids. Some liquids have partial, incomplete bonds that are in flux i.e. Water Metallic bonding. Im basicly just talking about pgf 1 of this wiki article. if your read the article, it gets complicated. Apparently metals even if solid are closer to liquids than Chrystal’s and of course gasses.
Gas- further dispersed entities i.e. Molecules/atoms
Importance of Metallic Bond
Metallic bonds allow the elements to conduct electricity, they can be formed into shapes and they conduct heat easily. This is the strongest of the three major bonds because the electrons are shared in more than just the first shells. The more shells involved in sharing electrons, the stronger the bond.
Hydrogen Bond
An atom of hydrogen linked covalently to a strongly electronegative atom can establish an extra weak attachment to another electronegative atom in the same or different molecules. This attachment is called a hydrogen bond. To distinguish from a normal covalent bond, a hydrogen bond is represented by a broken line eg X – H…Y where X & Y are two electronegative atoms. The strength of hydrogen bond is quite low about 2-10 kcal mol–1 or 8.4–42 kJ mol–1 as compared to a covalent bond strength 50–100 kcal mol–1 or 209 –419 kJ mol–1
Conditions for Hydrogen Bonding
Hydrogen should be linked to a highly electronegative element.
The size of the electronegative element must be small.
These two criteria are fulfilled by F, O, and N in the periodic table. Greater the electronegativity and smaller the size, the stronger is the hydrogen bond which is evident from the relative order of energies of hydrogen bonds.
Types of Hydrogen Bonding
Intermolecular hydrogen bonding:This type of bonding takes place between two molecules of the same or different types. For example,
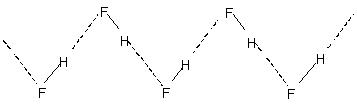
Intermolecular hydrogen bonding leads to molecular association in liquids like water etc. Thus in water only a few percent of the water molecules appear not to be hydrogen bonded even at 90°C. Breaking of those hydrogen bonds throughout the entire liquid requires appreciable heat energy. This is indicated in the relatively higher boiling points of hydrogen bonded liquids. Crystalline hydrogen fluoride consists of the polymer (HF)n. This has a zig-zag chain structure involving
H-bond.
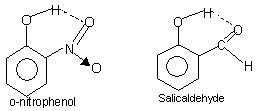
Intramolecular hydrogen bonding: This type of bonding occurs between atoms of the same molecule present on different sites. Intramolecular hydrogen bonding gives rise to a closed ring structure for which the term chelation is sometimes used. Examples are
o-nitrophenol, salicylaldehyde.
Importance of Hydrogen Bonding in Biological Systems
Hydrogen bonding plays a vital role in physiological systems. Proteins contain chains of amino acids. The amino acid units are arranged in a spiral form somewhat like a stretched coil spring (forming a helix). The N-H group of each amino acid unit and the fourth C=O group following it along the chain, establishes the N–H—O hydrogen bonds. These bonds are partly responsible for the stability of the spiral structure. Double helix structure of DNA also consists of two strands forming a double helix and are joined to each other through hydrogen bond.
Effect of Hydrogen Bonding
Hydrogen bonding has got a very pronounced effects on certain properties of the molecules. They have got effects on
- State of the substance
- Solubility of the substance
- Boiling point
- Acidity of different isomers
These can be evident from the following examples.
Example. H2O is a liquid at ordinary temperature while H2S is a gas although both O and S belong to the same group of the periodic table.
Solution: H2O is capable of forming intermolecular hydrogen bonds. This is possible due to high electronegativity and small size of oxygen. Due to intermolecular H-bonding, molecular association takes place. As a result the effective molecular weight increases and hence the boiling point increases. So H2O is a liquid. But in H2S no hydrogen bonding is possible due to large size and less electronegativity of S. So it’s boiling point is equal to that of an isolated H2S molecule and therefore it is a gas.
Example.Ethyl alcohol (C2H5OH) has got a higher boiling point than dimethyl ether (CH3-O-CH3) although the molecular weight of both are same.
Solution: Though ethyl alcohol and dimethyl ether have the same molecular weight but in ethyl alcohol the hydrogen of the O-H groups forms intermolecular hydrogen bonding with the OH group in another molecule. But in case of ether the hydrogen is linked to C is not so electronegative to encourage the hydrogen to from hydrogen bonding.
Due to intermolecular H-bonding, ethyl alcohol remains in the associated form and therefore boils at a higher temperature compared to dimethyl ether.
Credit:https://www.askiitians.com/iit-jee-chemical-bonding/metallic-and-hydrogen-bonding.html


Leave an answer
You must login or register to add a new answer.